Abstract
Background: Follicular lymphoma (FL) is typically associated with the BCL-2 chromosome translocation t(14:18), which leads to overexpression of the intracellular anti-apoptotic protein BCL-2. In addition, the B-cell receptor (BCR) promotes development, proliferation, and survival of normal B-cells, and is frequently manipulated in B-cell malignancies. The BCL-2 family are hypothesized to confer resistance to venetoclax. These proteins are up-regulated by the tumor microenvironment but down-regulated with ibrutinib. Xenograft models have shown that venetoclax + obinutuzumab demonstrates significantly increased anti-tumor activity compared with the agents individually. Finally, the combination of obinutuzumab, venetoclax, and ibrutinib has been shown to be safe in prior phase I trials in CLL and mantle cell lymphoma. We hypothesized that the combination of obinutuzumab, venetoclax, and ibrutinib would result in synergism enhancing their efficacy, and report our preliminary results here.
Methods: We conducted a phase II, open-label trial with a planned sample size of 40 patients involving patients ≥18 years old with previously untreated FL. Obinutuzumab 1000 mg IV was given on days 1, 8 (± 2 days), and 15 (± 2 days) of cycle 1, day 1 (± 7 days) of cycles 2 through 6, then on day 1 (± 7 days) of cycle 8, 10, 12, 14, 16, 18, 20, 22, and 24 of cycles 7-24. Venetoclax was started on day 4 (± 2 days) of cycle 1 at 400 mg and continued once daily through cycle 24 without a ramp up. Ibrutinib was given at 560 mg on day 1 (± 2 days) of cycle 1 and continued daily through cycle 24. Cycles were 28 days. Primary endpoint was complete response rate (CRR), determined by PET/CT based on Lugano 2014 criteria, as assessed by the investigator. Secondary endpoints included overall response rate (ORR), duration of response (DoR), and treatment-emergent adverse events (TEAEs). DoR was defined as time of first response, either a partial or complete response, to progression, death, or date of last follow-up.
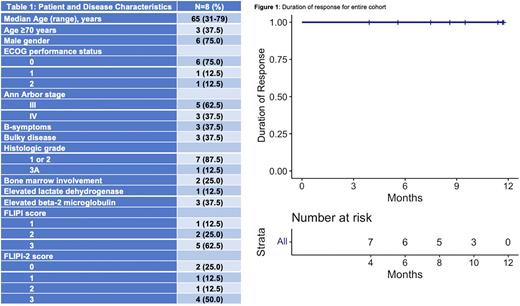
Results: 8 patients were enrolled and underwent response evaluation as of July 20,2022. The median follow-up was 12.2 months (IQR, 11.3;14.3). Patient and disease characteristics are summarized in Table 1. The CRR and ORR were each 100%. 6/8 patients achieved CR upon first response assessment after 3 cycles, 1 patient achieved CR upon first response assessment after 2 cycles, and 1 patient achieved a partial response upon first response assessment after 3 cycles and a CR upon second response assessment after 6 cycles. The median DoR was not reached (Figure 1). The median number of cycles completed was 9 (range, 6-15).
The TEAEs observed were fatigue [grade 3 (n=3), grade 1 (n=2)], lymphopenia [grade 3 (n=2), grade 2 (n=1)], diarrhea [grade 2 (n=1)], neutropenia [grade 3 (n=1)], rash [grade 1 (n=1)], and thrombocytopenia [grade 4 (n=1)] . One patient required a dose reduction in venetoclax to 400 mg on days 1-10 of each cycle 222 days after starting treatment due to grade 3 fatigue, and one patient with a history of immune thrombocytopenic purpura (ITP) required multiple dose reductions in both venetoclax and ibrutinib for recurring grade ≥3 thrombocytopenia and was eventually taken off study for this reason 241 days after starting treatment. Two other patients discontinued therapy early due to patient choice. There were no other treatment-related discontinuations. The other 5 patients remain on treatment.
Conclusion: Although the sample size and duration of follow-up were limited, the combination of obinutuzumab, venetoclax, and ibrutinib demonstrated remarkable efficacy and is a promising non-chemotherapeutic alternative for untreated FL. Non-hematologic toxicities were manageable, and no hematologic toxicity led to clinical complications.
Disclosures
Rosenberg:Takeda: Other: Institutional Research; Kangpu: Other: Institutional Research; Bristol Myers Squib: Research Funding; Adaptive: Consultancy; Janssen, Takeda: Speakers Bureau. Timmerman:Bristol Myers Squibb: Research Funding; Merck: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Oncovalent: Consultancy; A2: Consultancy. Heyman:Oncternal Therapeutics, INC: Research Funding; Epizyme, INC: Honoraria; Beigene: Honoraria; Aztrazeneca: Research Funding. Abdulhaq:Genentech: Membership on an entity's Board of Directors or advisory committees; Alexion: Speakers Bureau; Oncopeptide: Speakers Bureau; Jansen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tuscano:Genentech: Research Funding; Pharmacyclics: Research Funding; Takeda: Research Funding; Achrotech: Research Funding; ADC therapeutics: Research Funding; BMS: Research Funding; Celgene: Research Funding.
OffLabel Disclosure:
Venetoclax and ibrutinib is not approved in follicular lymphoma
Author notes
Asterisk with author names denotes non-ASH members.